Describe Some of the Chemical Effects of Acids and Bases
Some acids are strong electrolytes because they ionize completely in water yielding a great many ions. Some weak acids and bases are used in foods for example vinegar is a base and citric acid From lemons etc.

Acids And Bases Boundless Chemistry
Describe the pH scale and its relationship to acidic and basic solutions.
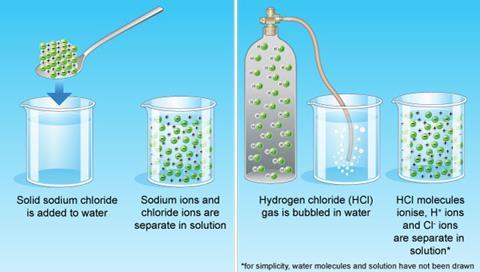
. Acids have a pH lower than 7. It can conduct electrical current. What are the properties of acids and bases.
Bases turn phenolphthalein to purple Acids neutralize bases producing a salt and water. Acid burns usually cause immediate pain and tissue damage. Acids turn blue litmus to red 2.
Bases turn methy l orange to yellow 3. Acids turn methyl orange to red Bases effect indicators. Hydrochloric Acid HCl Hydrochloric acid is the strong acid which is found inside our body in the gastric juice.
However over the course of the history of chemistry it took centuries to understand these substances fully. Please respond if this did not fully help you understand acids. It has a pH level of more than 7.
How acidic or alkaline a substance is the pH of the substance can be measured using the pH scale a continuous range that stretches from below 0 to above 14. Base has a characteristic to neutralize acid. It transforms red litmus paper into blue.
Lets check its everyday uses. Acids and bases can be defined via three different theories. When the metal iron disappears in an acid solution we can describe the acid as being what.
Hydrofluoric acid penetrates the skin rapidly and may even result in bone damage. Here are the names of some of the common acids and bases and the formulas associated with them. Many acids have a sour taste.
Other acids are weak electrolytes that exist primarily in a non-ionized form when dissolved in water. Concentrated acids and bases are corrosive and cause chemical burns if they come into contact with the skin eyes or internal organs. Proton A subatomic particle that is one of the basic building blocks of the atoms that make up matter.
Acids and bases are used in many chemical reactions. Water which has both acid and base is considered chemically neutral having a pH of 7. Is obviously an acid.
The farther above 7 the stronger the base. Acids and bases have some general properties. The Bronsted-Lowry theory defines an acid as a proton donor and a base as a proton acceptor.
Where are acids and basis commonly used. Some common acids are hydrochloric acid nitric acid carbonic acid and acetic acid. Acids react with bases to produce a salt compound and water.
Mixing equal amounts of a. The body has many molecules and compounds called acids and bases that can alter body functions. It has a bitter taste.
Bases make solutions that are slippery. Citric acid found in oranges and lemons is one example in which the sour taste is related to the fact that the chemical is an acid. The farther from 7 the stronger the acid.
Lactic acid-acid found in yogurt butter and milk. Bases turn red litmus to blue 2. Sour vinegar Bitter baking soda Smell.
A base is a substance with a pH value greater than 7. Ranging in pH scale from 0 to 7. They can be easily identified by their taste that is acids taste sour and bases taste bitter and salts itself have salty taste.
Alkaline solutions also called bases have a pH higher than 7. Explain the importance of buffers in organisms. The name acid gives us sensory images of Sourness.
Molecules that are bases usually have a bitter taste like caffeine. What is released when an acid is dissolved in water. A pH of 7 is perfectly neutral.
Turns litmus paper red. Can eat through some metals. Acids bases and salts affect chemistry as well as our day to day life.
It helps in the breakdown of all. They are the chemical substances. Bases have a pH greater than 7 and can accept a proton or produce an OH - ion in a reaction.
An acid is a proton donorBecause a hydrogen atom without its electron is a proton any substance that releases hydrogen ions H. Acids are common chemicals and can be found everywhere even in our food. Acids Bases Taste sour Taste Bitter pH less than 7 pH greater than 7Examples of acids.
Lemons vinegar and sour candies all contain acids. Some can burn skin. Acids are chemicals with a pH less than 7 that can donate a proton or H ion in a reaction.
Acids and bases pervade our lives from the laboratory to the kitchen and these crucial substances are used as laboratory reagents industrial catalysts food additives and in cleaning products. Acids change the color of certain acid-base indicators. React with many oils and fats.
Frequently react with metals to form H 2. Bases are termed alkaline meaning that they neutralize acids. Determine whether a solution is acid basic or neutral.
The Arrhenius theory of acids and bases states that an acid generates H ions in a solution whereas a base produces an OH ion in its solution. Acids have a sour taste. Examples of Acids and Bases in Everyday Life.
When equal moles of an acid and a base are combined the acid is neutralized by the base. Usually no smell except NH 3 Texture. 7 rows Tertiary amines and bulky secondary amines are poorer nucleophiles and behave.
4 properties of acids. They are responsible for most color change reaction and are used to adjust the pH of chemical solutions. An acid is a solution with a value between 1 and 699 on the pH scale a chemical gauge of acidity and alkalinity that goes from 1 to 14.
The products of this reaction are an ionic compound which is labeled as a salt and water. If you mix equal amounts of a strong acid and a strong base the two chemicals essentially cancel each other out and produce a salt and water. Acids and bases The pH scale.
It keeps blue litmus paper blue. In contrast you may not notice right away if your skin contacts a base.

Acids And Bases Chapter 14 Acid Base Chemistry Acid Base Chemistry Characteristics Acidsbases Ph Less Than 7ph Greater Than 7 Donates H Ionsaccepts Ppt Download

Acid Base Reaction Definition Examples And Uses
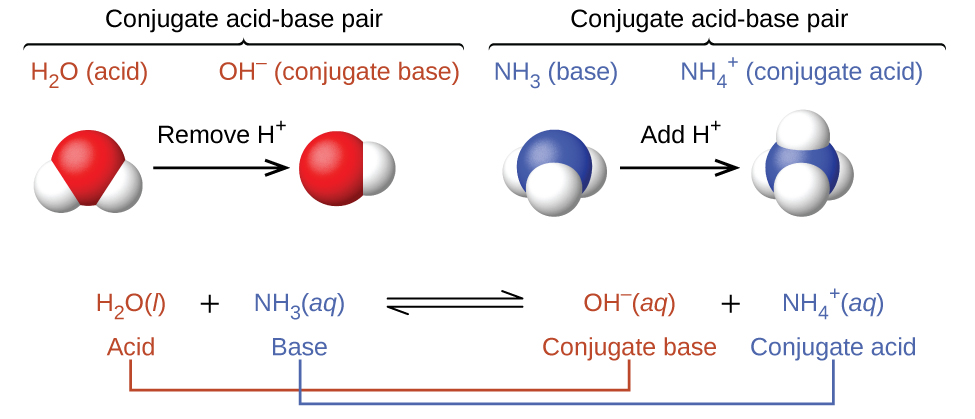
14 1 Bronsted Lowry Acids And Bases Chemistry
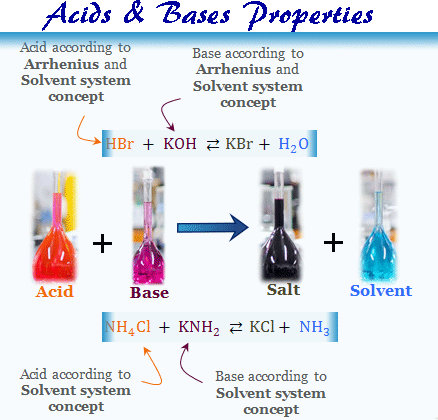
Acids Bases Properties Definition Concept Theory Examples
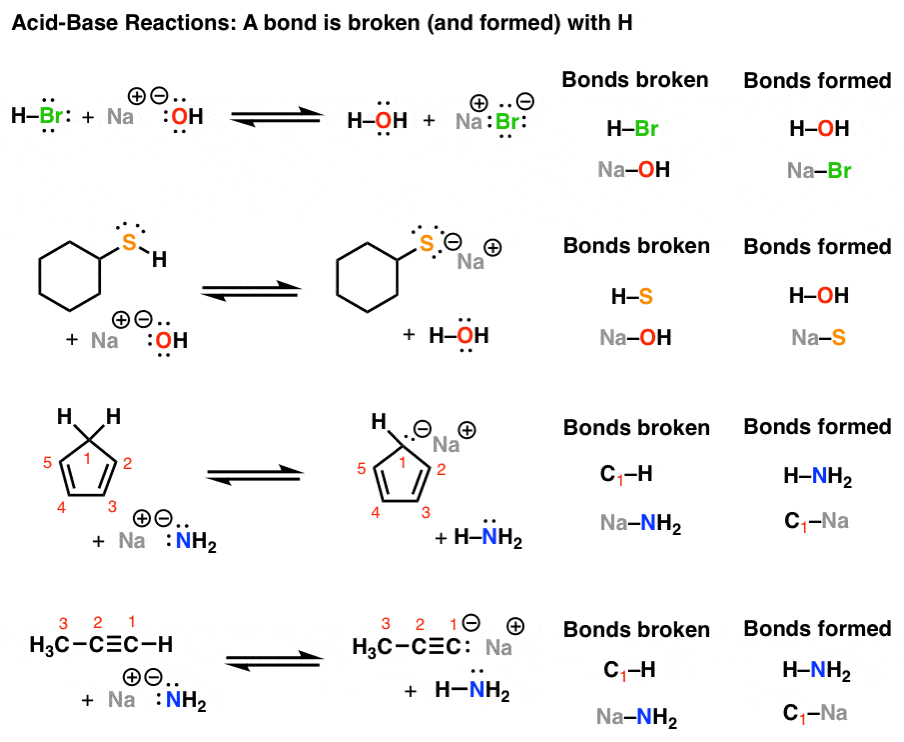
Introduction To Acid Base Reactions Master Organic Chemistry

Acid Base Reaction Definition Examples And Uses
7 8 Acids And Bases In Industry And In Daily Life Chemistry Libretexts
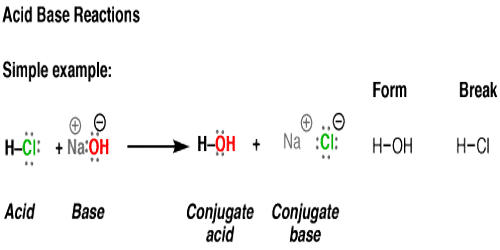
Acid Base Reaction Assignment Point
Acids And Bases Basic Introduction Chemistry Youtube
Acid Base Chemistry Chemistry Encyclopedia Reaction Water Metal Gas Number Equation Salt Property

Acids And Bases Definition Examples Properties Uses With Videos Faqs

Properties Of Acids And Bases Physical And Chemical Properties With Examples
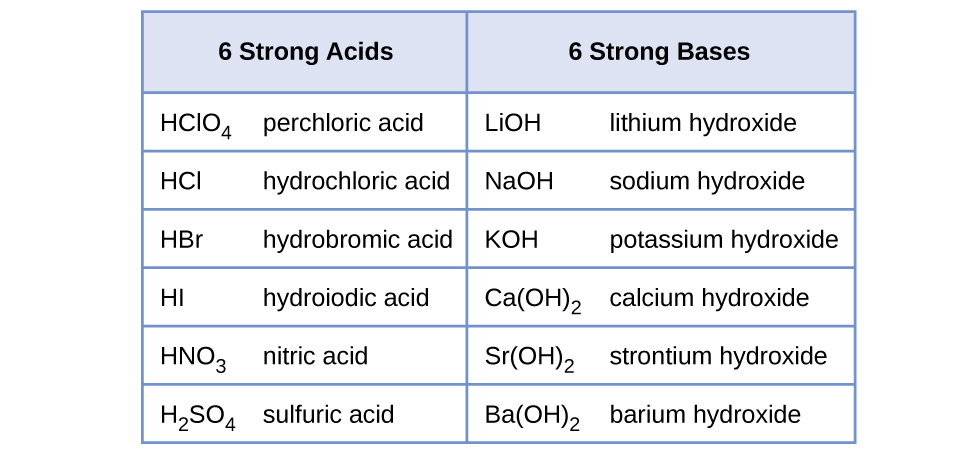
14 3 Relative Strengths Of Acids And Bases Chemistry

Acid Base Reactions In Organic Chemistry Master Organic Chemistry

Relative Strengths Of Acids And Bases Chemistry For Majors

Chemistry What Are Acids And Bases Shmoop Chemistry

Acids And Bases Cpd Rsc Education

Organic Acids And Bases Chemistry Steps

Chemistry Acid Base Reaction Chemical Equations 13 Of 38 Types Of Reactions Vii Youtube

Comments
Post a Comment